Entrepreneurship & Strategy
Cosmetics regulations in Europe, the US and the GCC: similarities and differences?
What are the similarities and differences between cosmetics regulations in Europe, the US and the Gulf States?
European Regulation 1223/2009, MoCRA, GSO 1943:2021
A brand launching in 2025 needs to take international cosmetics regulations into account. Knowing the regulatory specificities of each region means anticipating future international expansion right from the development phase. Here's an overview of the similarities and specificities of cosmetics regulations in each region. Europe, the US and the Gulf States.
European cosmetics regulations - Regulation 1223/2009
European cosmetics regulations: the concept of Responsible Person and DIP
In Europe, the regulation of cosmetic products is based on Regulation (EC) No. 1223/2009, which requires that each product placed on the market be accompanied by a Product Information Package (PIP). The DIP includes all the information needed to guarantee product safety and conformity. An essential element of the European regulation is the concept of the "responsible person" (RP), who is a legal entity responsible for placing the cosmetic product on the market and ensuring its compliance with European legislation.
Who is the Responsible Person (RP) ?
Visit person responsible is responsible for ensuring the conformity of cosmetic products before they are marketed. According to Regulation 1223/2009 :
-
Manufacturer If the manufacturer is located in the European Union, he is responsible for marketing the cosmetic product.
-
Importer If the manufacturer is located outside the EU, theimporter becomes the person in charge in Europe.
-
Distributor The distributor can also be designated as the responsible person if the manufacturer or importer does not assume this responsibility. If the manufacturer is a subcontractor of a brand, the client (the brand) is the responsible person.
In Europe, the person responsible must register the product on the Cosmetic Product Notification Portal (CPNP) before putting it on the market.
Contents Product Information Package (PIP) in Europe :
Visit DIP must contain the following information:
-
Complete formulation of the product, including list of ingredients and information on raw materials.
-
Safety assessment report of the finished product (including stability tests, microbiological challenge test, and skin tolerance evaluation).
-
Manufacturing file with Good Manufacturing Practice (GMP), in particular compliance with standard ISO 22716.
-
Labelling and claims on the product, which must be justified by scientific evidence. (find out more about labelling requirements for the European market)
-
Test report performed to guarantee product safety (stability tests, microbiological tests, etc.).
Visit Product Information File is essential to ensure that the product complies with European standards and can be safely placed on the market.
Identification of Responsible Person (RP) on Packaging in Europe:
In Europe, the person responsible must be clearly identified on the packaging of cosmetic products. The name and address of the responsible person must be indicated legibly and visibly on the product packaging or label. This enables consumers, health authorities and other stakeholders to know who is responsible for product safety and compliance.
Cosmetics regulation in the United States: MoCRA and its changes
In the United States, the Federal Food, Drug, and Cosmetic Act (FDCA) governs the safety of cosmetic products, but the recent Modernization of Cosmetic Regulation Act (MoCRA), which comes into force in 2022, has tightened requirements, particularly in terms of notification, GMP and product safety.
Who is the Responsible Person (RP) in the United States?
Under MoCRA, the responsible person may be the manufacturer or distributor of cosmetic products. This person is responsible for marketing and ensuring product compliance. Under MoCRA, the responsible person must notify the FDA within 60 days of marketing. The safety certificate required under MoCRA is similar to the safety assessment in Europe, although it is not as detailed in terms of toxicology.
Visit FDA notification file includes the following items:
-
Company name and address (Responsible person)
-
List of ingredients with concentrations.
-
Description of the product and its intended use.
- GMP compliance
-
Safety certificate, confirming that the product complies with safety standards, including risk assessments and stability tests.
-
FDA-compliant labeling, including claims (which must be verifiable).
Identification of Responsible Person (RP) on Packaging in the United States :
In the USA, MoCRA also requires that the identity of the responsible person (company name and address) be clearly indicated on cosmetics packaging. This ensures that, in the event of a problem, the authorities can quickly contact the responsible person to resolve the situation.
Gulf Cosmetics Regulations (GCC): GSO 1943:2021 and local registrations
The Gulf Cooperation Council (GCC), which includes Saudi Arabia, the United Arab Emirates, Kuwait, Bahrain, Qatar and Oman, uses a common regulatory framework, defined by Gulf Standard GSO 1943:2021, which governs the safety, quality and labeling of cosmetic products. However, each country imposes local product registrations.
Who is the Responsible Person (RP) in GCC countries?
The person responsible in GCC countries may be the manufacturer or importer of cosmetic products. As in Europe, the responsible entity must ensure that the product complies with local standards and submit products for registration with the competent authorities in each country.
DIP in GCC countries includes elements similar to those in Europe:
-
List of ingredients and concentrations in accordance with local standards.
-
Safety certificate, including stability tests, microbiological tests and a safety assessment by a qualified toxicologist.
-
Certificate of Good Manufacturing Practice (GMP), often in compliance with ISO 22716.
-
Labeling in accordance with local requirements, which may include information in Arabic.
Local registration procedures in GCC countries :
In each GCC country, products must be registered before they can be marketed, and the steps involved may differ slightly from one country to another. In Saudi Arabia, products must be registered with the Saudi Food and Drug Authority (SFDA), while in the United Arab Emirates, registration is with the Ministry of Health or local authorities such as the Dubai Municipality.
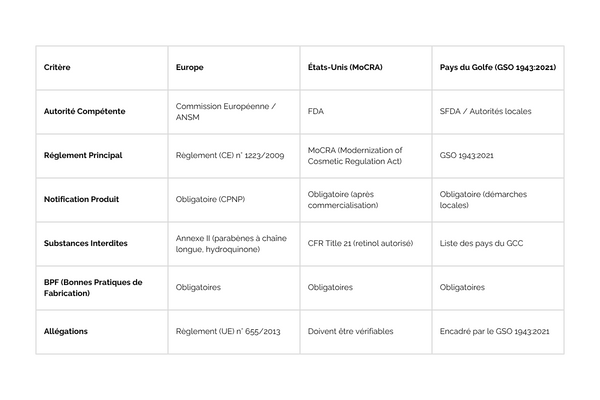
What the various cosmetics regulations have in common
- Good Manufacturing Practices (GMP): Required in all regions (ISO 22716).
-
Identification of the person responsible for the packaging
-
Safety assessment of finished products: Testing focuses on the finished product, not on individual ingredients. This includes stability, tolerance and microbiology tests.
-
Prohibited substances: Each region has a list of banned and restricted substances, although these lists vary slightly. Hydroquinone and certain long-chain parabens are banned in all three regions.
The differences between cosmetics regulations
-
Advance product notification :
-
In Europe, notification via the CPNP is mandatory prior to marketing.
-
In the United States, post-marketing notification to the FDA is mandatory with MoCRA.
-
In GCC countries, local registration is required before marketing, but each country has its own specific procedures.
-
-
Banned and restricted substances :
-
In Europe, Annexes II and III of Regulation 1223/2009 detail the banned and restricted substances. Examples include hydroquinone and long-chain parabens.
-
In the USA, CFR Title 21 lists prohibited substances, but there are fewer specific restrictions than in Europe. Retinol is authorized under specific conditions.
-
GCC countries follow GSO 1943:2021, but lists of prohibited ingredients may vary slightly from country to country.
-
Entrepreneur, product manager, beauty professional? Sign up to receive our technical updates and inspirations.
Conclusion
Regulations in the Gulf States and the United States are largely based on European regulations, offering a considerable advantage to companies in the European Union, which are already well acquainted with these requirements since the entry into force of the Regulation (EC) no. 1223/2009 in 2009. Because of this similarity, European companies have an important advantage in navigating product safety, notification and claims control requirements more easily, facilitating their expansion into international markets while minimizing the adjustments needed to comply with local regulations. If you need support, please contact us here.
KEYWORDS
Similarities and differences between cosmetics regulations worldwide